Avelumab is a recently developed drug which has shown promising results for pleural and peritoneal mesothelioma patients. The first phase of the clinical trial was completed at the beginning of 2016 and the results for unresectable malignant mesothelioma were subsequently presented by Dr. Raffit Hassan of the National Cancer Institute at the American Society of Clinical Oncology Annual Meeting in Chicago.
The study included 1,600 patients suffering from various forms of cancer, out of which 53 diagnosed with stage III or IV pleural or peritoneal mesothelioma. Their cancer was very advanced and thereby, the tumors could not be safely removed surgically. All mesothelioma patients involved in the clinical trial had previously undergone platinum or pemetrexed chemotherapy. However, treatment was ineffective, as their condition did not cease to progress. The entire mesothelioma cohort had a life expectancy of approximately 3 months and was not previously exposed to T-cell targeting or anti-PD-1/PD-L1 antibodies. Additionally, patients had to discontinue all types of cancer treatment 28 days prior to partaking in the clinical study.
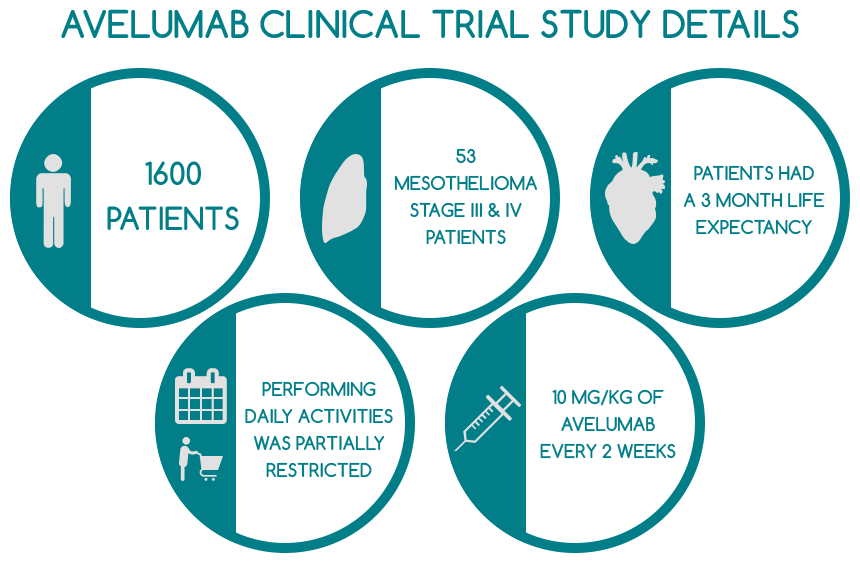
The average duration of the treatment was 12 weeks, the mesothelioma patients’ median age was 66 and they also had an ECOG performance status of 1, which means their functioning in terms of performing daily activities and taking care of themselves without the aid of other people was partially restricted. Approximately 30% of all patients had received one to three different treatments for cancer before participating in this study.
Mesothelioma patients were administered 10 milligrams of Avelumab per kilogram every two weeks. Some of the most relevant findings of the clinical trial in respect to mesothelioma are:
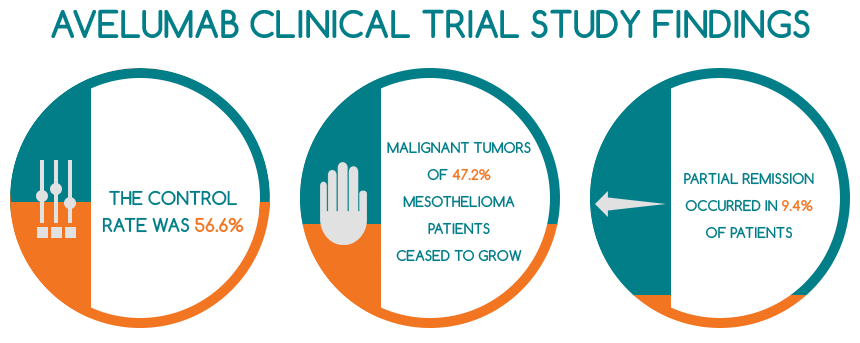
- the control rate was 56.6% and the malignant tumors of twenty-five mesothelioma patients (47.2% of the entire cohort) treated with Avelumab ceased to grow
- partial remission occurred in 9.4% of them
- 77.4% of patients experienced mild side effects such as fatigue, fever and chills and severe adverse effects affected only five of them
- the average duration of response could not be properly assessed
- no deaths related to the Avelumab treatment ensued
Avelumab, an anti-PD-L1 drug, is used in immunotherapy, a treatment approach whose purpose is to improve the body’s immune system so that it can efficiently fight cancer. PD-L1 (programmed death ligand 1) is a protein present on the surface of certain malignant cells which suppresses immune responses and prevents the body from activating T cells. Consequently, the tumor continues to develop, as the immune system is too weakened to manage its growth. The drug inhibits the further development of mesothelioma tumors by controlling the activity of the PD-L1 protein. Thus, the immune system is considerably boosted and is able to counteract cancer more efficiently.
Merck and Pfizer, two world-renowned pharmaceutical companies, united to develop Avelumab and initiated the JAVELIN program, which provides cancer patients, including those diagnosed with pleural and peritoneal mesothelioma, with experimental treatments. The drug is still under research and the estimated time of the clinical trial’s completion is May 2018. The ultimate goal of the JAVELIN program is to accurately evaluate the safety, tolerability and appropriate dosage of Avelumab. Mary Hesdorffer, Executive Director of Mesothelioma Applied Research Foundation, concluded that “Though it was a negative study, there is a role for PD-L1 inhibitors in mesothelioma.”
If you or a loved one suffers from mesothelioma, we strongly encourage you to seek experimental treatments which might considerably improve your prognosis or even lead to the remission of cancer, provided it is not very advanced. We have compiled a list of ongoing clinical trials you can sign up for. You can easily find out what clinical trials are available for your specific condition, what drugs are involved in each of them, as well as the current phases of the programs.

 January 27, 2017
January 27, 2017 By
Stan Gottfredson
By
Stan Gottfredson  Read by 1073 Users
Read by 1073 Users